
Recently, Professor Mai Liqiang’s research team has achieved significant breakthroughs in the study of aqueous zinc-ion batteries. Their findings, titled “Organic-solvent-free primary solvation shell for low-temperature aqueous zinc batteries,” were published in Chem, the sub-journal of Cell. Wuhan University of Technology (WUT) is the solely affiliated institution, with PhD candidate Geng Lishan as the first author. Professors Mai Liqiang, Zhou Liang, and Distinguished Researcher Meng Jiashen are co-corresponding authors.
Aqueous zinc-ion batteries (ZIBs) are regarded as a promising alternative to lithium-ion batteries for large-scale energy storage due to their high safety and low cost. However, the formation of zinc metal dendrites and the narrow operating temperature range of aqueous electrolytes have hindered further development of ZIBs. Introducing organic solvents into aqueous electrolytes to form hybrid electrolytes has been shown to partially mitigate these issues. However, in traditional hybrid electrolytes, the organic solvent molecules exhibit a stronger affinity for zinc ions compared to water molecules in the primary solvation shell. This leads to slow desolvation kinetics, especially at low temperatures, resulting in significant electrochemical polarization, reduced coulombic efficiency in zinc deposition/stripping, dendrite accumulation, poor cycling stability, and limited rate performance. Therefore, developing hybrid aqueous electrolytes that exhibit rapid desolvation kinetics even at low temperatures is critical to advancing clean energy technologies and supporting the contributing to national carbon neutrality goals.
To address these challenges, Professor Mai’s team designed a hybrid aqueous electrolyte with a primary solvation shell free of organic solvent molecules. This innovation overcomes the slow desolvation kinetics of traditional hybrid electrolytes, particularly at low temperatures. As a representative example, the designed electrolyte (ZAD4) consists of 1 M zinc acetate (Zn(Ac)2, high lattice energy) solution and 40 vol% dipolar aprotic solvent dimethyl sulfoxide (DMSO). The Zn2+ ions coordinate with CH3COO⁻ and H2O to form Zn2+(CH3COO-)2(H2O)4 clusters. Due to the strong hydrogen bonding between CH3COO⁻ and H2O, DMSO does not participate in the primary solvation shell, thanks to its non-solvating effect on CH3COO⁻ and the hydrogen bonding between DMSO and H2O. This weak solvation structure promotes fast charge transfer kinetics and facilitates rapid Zn2+ migration through the gradient solid electrolyte interphase (SEI) generated by the desolvation of CH3COO⁻ and DMSO adsorption. At -20°C, the zinc anode exhibits an initial coulombic efficiency (ICE) of 97.7%, a long cycle life of up to 5,600 hours, a zinc depth of discharge (DODZn) of 50%, and a low overpotential. Additionally, a polyaniline (PANI)||Zn full battery based on this electrolyte, featuring a mixed CH3COO⁻ and Zn2+ insertion/extraction mechanism, demonstrates a low voltage polarization of 0.05 V, a Zn DODZn of 25%, a high capacity of approximately 160 mAh g-1 at 0.1 A g-1, and long-term cycling stability over 8,800 cycles with excellent rate performance. This design strategy can be extended to develop a series of similar solvation shell electrolytes.
Professor Mai Liqiang’s team has been engaged in research and development on energy storage batteries and key materials for years, contributing to their industrial applications. They pioneered the first universal model for in situ characterization of electronic/ionic transport in single nanowire devices and established the “Mai-Yan” field-effect energy storage theory, which regulates electrochemical reaction kinetics via dual continuous electronic/ionic transport. They have also advanced large-scale fabrication technologies for energy storage materials and devices. The team has published over 600 SCI papers, including three in Nature and one in Science, with more than 56,000 SCI citations. They have authored two monographs in both Chinese and English and hold over 160 national invention patents. Additionally, they have led more than 30 national-level projects, including major scientific instrumentation initiatives.
Since 2015, the team has focused on zinc-ion battery research, achieving self-lubrication of interlayer ion transport through pre-embedding solvent molecules, and developing dozens of cathode materials suitable for various applications. These innovations earned them the Excellence in Research Award from the International Electrochemical Energy Society. They also developed a 3D conductive framework composite anode material, addressing the challenge of simultaneously improving energy density and cycling stability, which garnered the Guanghua Engineering Award and the International Automotive Lithium Battery Association’s Excellence in Research Award. Moreover, their discovery of a eutectic composite electrolyte with a unique solvation structure led to the development of Ah-grade soft-pack batteries with wide operating temperature ranges, earning them the Ho Leung Ho Lee Foundation Award. The team has established the world’s first customized production equipment for aqueous zinc-ion batteries, achieving breakthroughs in the large-scale fabrication of energy storage materials and devices. These advancements have been successfully commercialized and applied in collaboration with over 30 companies, including Dongfeng and Huawei, making significant contributions to the high-quality development and self-reliance of China's energy storage industry.
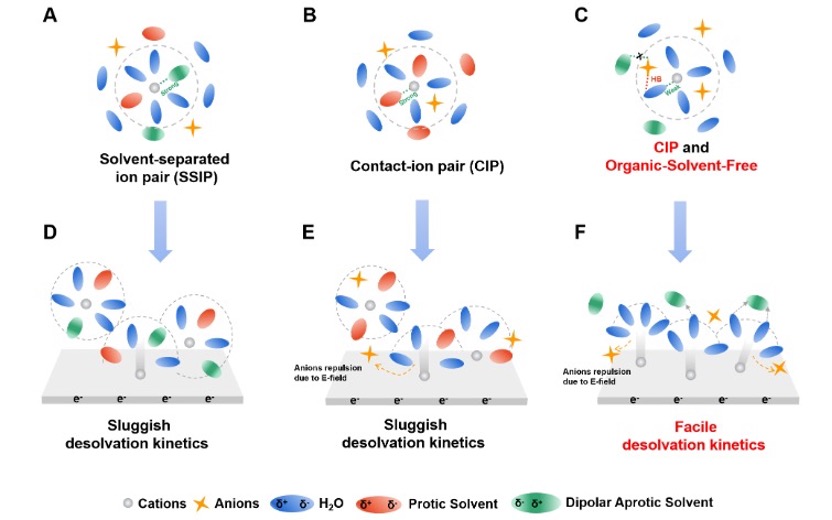
Figure 1: Primary solvation shell and desolvation behavior of traditional hybrid aqueous electrolytes compared to the electrolyte developed in this work.
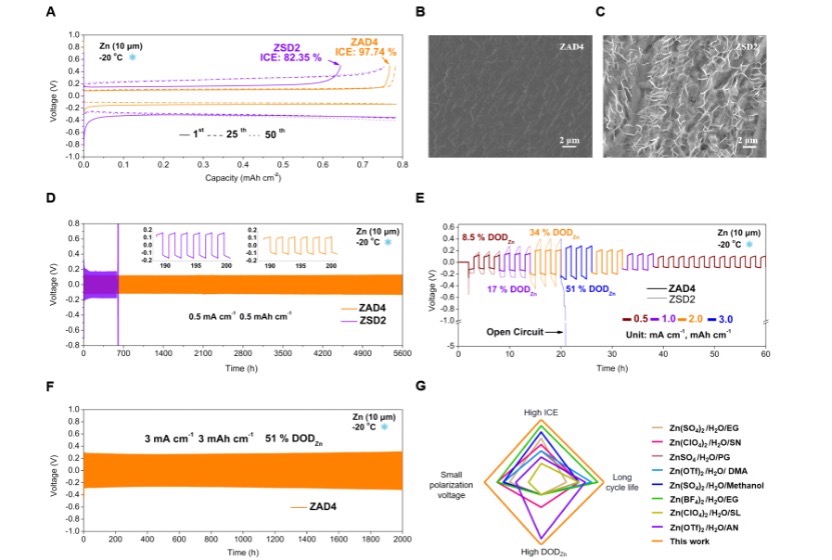
Figure 2: Electrochemical performance of the zinc anode at -20°C.
Paper link: https://doi.org/10.1016/j.chempr.2024.09.001
Written by: Geng Lishan
Rewritten by: Liang Muwei
Edited by: Wang Jingjing, Li Tiantian
Source: School of Materials Science and Engineering
|
|